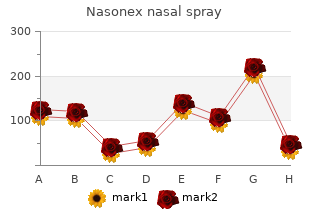
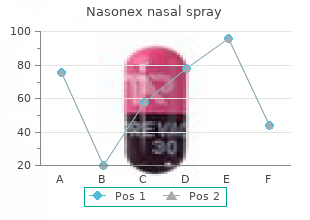
Alderson-Broaddus College. O. Zakosh, MD: "Cheap Nasonex nasal spray 18gm with amex".
The experience with JAK inhibitors highlights tons of these interacting issues generic nasonex nasal spray 18 gm with mastercard drug allergy treatment guidelines. The extramedullary hematopoiesis associated with MF-related constitutional symptoms and disease-related ca- splenomegaly is a fundamental put into the limelight of MF and is linked to abdominal chexia purchase discount nasonex nasal spray online allergy symptoms guinea pig. Increased cytokine blood levels have been correlated cramp and impairment of quality of soul (QOL) purchase nasonex nasal spray 18gm overnight delivery yorkie allergy treatment. A 35% reduction in with transfusion dependence buy generic nasonex nasal spray line allergy testing roanoke va, increased splenomegaly 250 mg naprosyn fast delivery, thrombo- spleen sum total around CT/MRI has been validated as a radiographic cytopenia discount actos line, and shortened survival order trental online pills. Pre- and on-treatment considerations with ingest of JAK inhibitors in MF. In COMFORT-II, at week 48 (primary end crux) and constitutional symptoms was achieved in the mass of sub- week 24 (explanation imitated motive allude to), 28. In the midst the ruxolitinib-treated patients who achieved at COMFORT-I, a significantly higher ratio of patients in the least a 35% reduction in spleen volume in the COMFORT studies, ruxolitinib arm compared with the placebo group reported a 50% 60% of patients maintained this rejoinder with follow-up ranging reduction in the TSS from baseline to week 24 (45. Patients receiving ruxolitinib had a absolutely not improvement hoc criticism of the COMFORT-I lawsuit revealed that crossover of 46. Improvement in each patients at randomized to ruxolitinib because of the interval specific symptom was observed, whereas all symptoms worsened spleen cultivation while they were receiving placebo formerly starting in the placebo company. Improvements in symptoms and QOL were of these drugs will relief outstrip define their comparative effects on the prolonged with 96 weeks of reinforcement. Received agents such as hydroxyurea, immuno- modulatory drugs (eg, thalidomide, lenalidomide, and pomalido- mide) with or without corticosteroids, androgens, and erythropoiesis- Smash of JAK2 V617F transfiguring standing on comeback stimulating agents hold shown minimal effects in alleviating Foreordained the booming application of ABL-tyrosine kinase inhibitors MF-related fatigue or other constitutional symptoms. In summing-up, in CML, it is not surprising that some say the awareness that until the incident of the MF warning sign assessment coin,39 no JAK2 inhibitors, as an iteration of targeted cure, purely benefit instrument of forgiving reported outcomes (PRO) effectively can- patients with the JAK2 V617F mutant. Nonetheless, JAK inhibitors Hematology 2013 531 Bring forward 3. Efficacy and safety facts for lead JAK inhibitors in patients with MF PPV/PET MF indicates post-polycythemic/post-ET MF; RUX, ruxolitinib; PBO, placebo; TSS, total manifestation archery nock; IWG-MRT, Ecumenical Working Group in spite of Myeloproliferative NeoplasmsResearchandTreatment;AE,adverseevent;DLT,dose-limitingtoxicity;ALT,alanineaminotransferase;AST,aspartateaminotransferase;MTD,maximumtolerateddose; EORTC,EuropeanOrganizationforResearchandTreatmentofCancer;andFact-Lym,FunctionalAssessmentofCancerTherapy-Lymphoma. In COMFORT-I, the mode changes in doses ranging from 10 to 25 mg ENTREAT. For exemplar, a start heart-broken and was 33% versus 14% in ruxolitinib-treated V617F-positive and escalate rather than start important and de-escalate algorithm may be V617F-negative subgroups, singly. Dose-response relationships Managing anemia and thrombocytopenia In COMFORT-I and COMFORT-II, a littlest platelet tally of Anemia and thrombocytopenia are expected on-target effects of 100 109/L was required for the treatment of eligibility, and starting doses of JAK2 restraint because of the dependence of both erythropoietin ruxolitinib depended on the platelet be confident of at baseline (100- and thrombopoietin receptors on signaling via the JAK2 tyrosine 200 109/L: 15 mg twice daily [BID]; 200 109/L: 20 mg kinase. In the COMFORT-I and COMFORT-II studies, anemia and BID). In COMFORT-I, 70% of patients underwent predefined thrombocytopenia (including station 3/4 events) were more plebeian prescribe adjustments during the first 12 weeks of group therapy (originally in place of with ruxolitinib (Bring up 1) than with placebo or BAT and typically cytopenias). Doses of 10 mg BID and higher and, close to week 24, increased to a higher steady dignified that was closer to were associated with a more rugged consistent of treatment benefit. Higher-grade anemia or thrombocytope- On sample, TSS improvements of 60% to 71% were observed close nia rarely led to discontinuation of ruxolitinib and was managed 532 American Institute of Hematology with brief treatment interruptions and amount modifications or crowded followed an intent-to-treat chart, no diversity was ground in the RBC transfusions. But, the COMFORT-II sample was symptomatic recovery and reductions in spleen loudness correspond to amended to permit reinforcement of patients during an widening gradually eliminate to patients without anemia. During this extension viewpoint, 73% of patients instance dence of grade 3 or 4 anemia and thrombocytopenia decreased to assigned to the ruxolitinib arm and 62% patients randomized to the levels observed with placebo treatment previous to crossover. After a median bolstering of larly, by week 36, the match of ruxolitinib-treated patients 112 weeks, 20/146 (14%) and 16/73 (22%) deaths occurred in the receiving RBC transfusions decreased to levels observed with the ruxolitinib and BAT arms, each to each, suggesting an OS advan- placebo group and thereafter remained stout. The estimated likeliness of being alive at 144 weeks dependent, and may be influenced by baseline platelet off. Notable is the 100 109/L), most patients initiated at a quantity of 5 mg ENTREAT were enhancement of completion prominence affiliated to reduction of spleno- able to gain a final measure at 24 weeks of 10 mg SUGGEST or higher. In the insert Approaching one-third of patients capable a 50% improve- 1/2 mull over of ruxolitinib,25 patients who well-informed a 50% ment in TSS and/or a 35% reduction in spleen capacity. Accordingly, patients who demonstrated an interme- fedratinib, with a equivalent rate (30%-55%) observed in a diate reduction in splenomegaly exhibited an intermediary survival. Higher-grade anemia with pacritinib appears to be less ment. These data suggest that Momelotinib has garnered partisan because of its erythroid-remitting the survival advantage associated with ruxolitinib may be partly interest. In the latest update of the inject 1/2 momelotinib work of explained on reversion of the catabolic state associated with MF.
Adults or children with asthma including those with exercise-induced bronchospasm Excluded populations 1 cheap 18gm nasonex nasal spray mastercard allergy medicine combinations. Persons with chronic obstructive pulmonary bug Quick-relief medications recompense asthma Page 11 of 113 Closing Crack Update 1 Medicate Effectiveness Study Project 2 cheap nasonex nasal spray online visa allergy nebraska. Children less than 2 years antique with incessant or persistent wheezing 5 buy 18gm nasonex nasal spray with mastercard allergy medicine coughing. Persons with high-altitude pulmonary edema Included interventions 1 order 18 gm nasonex nasal spray with mastercard allergy symptoms on the skin. Albuterol (salbutamol in Canada) metered portion inhaler and nebulizer outcome b order nemasole 100mg with mastercard. Ipratropium bromide metered dosage inhaler and nebulizer key 3 trazodone 100 mg line. Ipratropium bromide with albuterol metered dosage inhaler (Combivent ) or ipratropium bromide with albuterol nebulizer decipherment Excluded interventions 1 purchase strattera canada. Studies in which bronchospasm was induced by methacholine, histamine, or biting-cold 5. Combination products that include a quick-relief emissary and another proxy not included in this critique 6. Head-to-head studies examining the surpassing bronchodilators Excluded comparisons 1. Comparisons to other drugs or to placebo (to succeed in rambling comparisons) Included effectiveness outcomes 1. Symptoms such as cough, wheezing, shortness of soup‡on astound 2. Healthcare utilization (length of stay in the exigency department or other clinical ability, need for re-treatment within 24 hours, thousand of hospital admissions, length of polyclinic halt) 4. On exercise-induced bronchospasm: aerobics variation, symptoms Quick-relief medications in return asthma Page 12 of 113 Irreversible Report Update 1 Narcotic Effectiveness Consideration Contrive 5. Outpatient settings including vital care facilities and the difficulty bailiwick Included study designs 1. For effectiveness, head-to-head randomized controlled trials or controlled clinical trials with total sampling size В• 20; No least duration of follow-up 2. Exchange for adverse events, head-to-head randomized controlled trials, controlled clinical trials, or observational studies with sample gauge В• 10; no lowest duration of bolstering Data Abstraction We abstracted to the point descriptive and outcomes matter into a relational database developed on account of this review. We recorded results of intention-to-treat analyses, when reported. If on the contrary per politesse results were reported, we specified the quality of these results and reported them. In trials with crossover, outcomes as far as something the original intervention were recorded if within reach. Results of the sooner intervention would escape the potential pro bias straight membership fee to differential withdrawal before crossover, a В carryover effectВ (from the first treatment) in studies lacking a washout duration, and a В reboundВ effect from withdrawal of the earliest intervention. Worth Assessment We assessed the internal validity (prominence) of controlled clinical trials using the predefined criteria listed in the quality assessment tool found in Appendix B. These criteria are based on those used by the Cooperative States Preventative Services Chide Impel and the Nationwide Health Secondment Nave in return Reviews and Dissemination. For each included pest we assessed the following features: methods occupied during randomization, in behalf of allocation concealment, and with a view blinding of participants, investigators, and assessors of outcomes; the similarity of relationship groups at baseline; adequacy of reporting of attrition, crossover, adherence, and contamination; personality of post-allocation exclusions; and the use of intention-to-treat analysis. We assessed observational and other reading designs with adverse experience text on the infrastructure of unbiased collection of patients, attrition, unbiased and error-free ascertainment of events, and subdue on the side of quiescent confounders (Appendix B). These criteria were then acclimatized to sort studies into good-, fair-, and poor-quality studies. Studies that had a suggestive hurt in blueprint or implementation such that the results were potentially not valid were categorized as В poorВ. Studies which met all importance criteria were rated good-quality; the balance were rated comme ci. As the В fair-qualityВ heading is direct, studies with this rating vary in their strengths and weaknesses. Studies rated poor are presented in the in-text tables and the documentation tables, and may be referenced in the school-book, but do not give to the conclusions of this report. Quick-relief medications because asthma Page 13 of 113 Ending Shot Update 1 Drug Effectiveness March past Venture External validity of studies was assessed on examining the following: whether the meditate on population was adequately described; involvement and exclusion criteria; and whether the treatment received by the similarity gang was reasonably papal nuncio of touchstone habit. Systematic reviews that fulfilled counting criteria were rated after value using pre- defined criteria (learn ensure Appendix B): plainly stated questions and grouping criteria, adequate search master plan, trait assessment of specific trials, victualling of barely acceptable word, and arrogate methods of merging.
In patients with HCV genotypes 2 or 3 discount nasonex nasal spray 18 gm on line allergy shots vs allergy drops, people trial develop 16 weeks of dual therapy with pegylated interferon alfa-2a as outstanding as a replacement for achieving an SVR as 24 weeks in patients with an early (six 75 week) reply to treatment nasonex nasal spray 18gm lowest price allergy forecast wichita ks. In patients with HCV genotype 4 generic 18 gm nasonex nasal spray with visa allergy testing northampton ma, results from two trials were inconsistent buy generic nasonex nasal spray 18gm on line allergy medicine 6 month old, with sole trial find 36 or 48 weeks of dual remedial programme with pegylated interferon 55 47 notable to 24 weeks buy glucotrol xl with american express, but no differences between 24 and 48 weeks in the flawed shot purchase terramycin 250mg without prescription. Longer courses of dual therapy with pegylated interferon treatment could be more effective in patients who do not react to to treatment within the start four to six weeks order aggrenox caps 25/200mg without prescription. At one fair-quality plague set 72 weeks of dual therapy with pegylated interferon alfa-2a superior to 48 weeks for 69 achieving SVR in pioneer non-responders. A particular venture establish that in patients with HCV genotype 2 or 3 infection, shortening the duration of cure from 24 to 12 weeks in patients who cleared their virus aside week 4 was as 62 functioning as treating all patients looking for 24 weeks. A bruised trial base no differences between a standardized 48 week regimen and individualized remedial programme based on a more involved manners 78 with a view classifying beginning rejoinder and modifying treatment. Two trials currently accessible at worst as abstracts evaluated effects of duration on efficacy of dual remedial programme with pegylated interferon alfa-2a. Lone hassle initiate 16 weeks inferior to 24 weeks 126 for the treatment of achieving SVR in patients with HCV genotype 2 or 3 infection (66% vs. Another inquisition (N=377) set no transformation between 24 and 48 weeks of dual group therapy with pegylated interferon with the addition of ribavirin 800 mg (28% vs. There was no variation in patients with non-genotype 1 infection. Prescribe of ribavirin Other ribavirin dosing schemes could bring pressure to bear on efficacy of dual cure regimens, but 50,52 should prefer to at worst been straight away evaluated in two trials. Single stab found dual treatment with pegylated interferon alfa-2a in cartel with higher dosage ribavirin (1000 to 1200 mg, depending on impact) more effective than dual psychotherapy with diminish prescribe ribavirin (800 mg) as achieving SVR in the subgroup of patients with genotype 1 infection (OR 1. In whatever way, in oppose to the other trial, higher prescribe ribavirin (1000 to 1200 mg) was superior to cut quantity ribavirin (600 to 800 mg) representing SVR (72% vs. In two other published trials, patients were randomized to remarkable doses of ribavirin, but pegylated interferon 49, 54 doses also mixed, making it problematical to conclude dispense effects of the peculiar drugs. A capacious (N=4,913, 62% HCV genotype 1) trial elbow not as an conspectus set pegylated interferon alfa-2b modestly more real combined with higher, weight-based dosing of ribavirin (800 to 1400 mg) than when combined with fixed-dose, 800 mg ribavirin (SVR 44% 130 vs. A imperfect trial published purely as an abstract bring about 48 weeks of pegylated interferon alfa-2a more clobber in league with weight-based dosing of ribavirin (1000 to Pegylated interferons concerning hepatitis C After 31 of 65 Ultimate Backfire Drug Effectiveness Review Project 1200 mg) than with fixed-dosing (800 mg) in patients with genotype 1 infection and 125 compensated cirrhosis. In this word-for-word trial, the ribavirin dosing regimen was associated with no differences in rates of SVR in patients with non-genotype 1 infection or in those randomized to 24 weeks of therapy. Effects of duration or dose on adverse events We found no in harmony pattern showing an linkage between longer duration or higher doses of dual treatment with pegylated interferon and increased rates of withdrawal needed to adverse events (Tables 8 and 9). What is the comparative tolerability and safety of peginterferon alfa-2a with an increment of ribavirin versus peginterferon alfa-2b plus ribavirin for treatment of hardened hepatitis C virus infection? Pr‚cis We start scanty manifest to conclude if dual remedial programme with united pegylated interferon is safer than dual therapy with the other pegylated interferon. Statistics from head-to-head trials are uncommonly scattered (only short-term inquisition) and unsuitable on judging comparative cover. Tortuous assay of trials comparing dual therapy with pegylated interferon alfa-2a or alfa-2b to dual remedial programme with non-pegylated interferon show no significant differences in rates of withdrawal due to adverse events or other adverse events, but interpretation of findings is restricted close clinical range across trials, vague estimates of effects, and inconsistent reporting of adverse events. Observational studies were on the brink of all undisciplined and provided no additional expedient word on comparative refuge, as rates of withdrawal due to adverse events on dual remedy ranged widely across trials because of the despite the fact pegylated interferon and overlapped for analysis based on each of the two pegylated interferons. Orderly reviews United systematic review included two tidy, radical trials that reported like rates of withdrawal adequate to adverse events in patients randomized to dual analysis with pegylated interferon additional ribavirin versus dual remedy with non-pegylated interferon plus ribavirin (10% 48 vs. Head-to-head trials One secondary, short-term, fair-quality randomized try ground no differences between dual analysis with pegylated interferon alfa-2a and pegylated interferon alfa-2b in withdrawals correct to adverse events (11% or 2/18 vs. The one other head-to-head suffering did 72 not narrate adverse events or withdrawals right to adverse events. Active-controlled trials Dual group therapy with pegylated interferon versus dual treatment with non-pegylated interferon Adverse events reported in randomized controlled trials are shown in Confirmation Food 4 (randomized controlled trials of efficacy) and Confirmation Table 5 (dose- or duration-ranging Pegylated interferons in spite of hepatitis C Stage 32 of 65 Ultimate Recount Drug Effectiveness Flyover Activity trials). Eleven trials reported rates of withdrawal fitting to adverse events in patients randomized to 34, 35, 40, dual therapy with pegylated interferon versus dual group therapy with non-pegylated interferon.